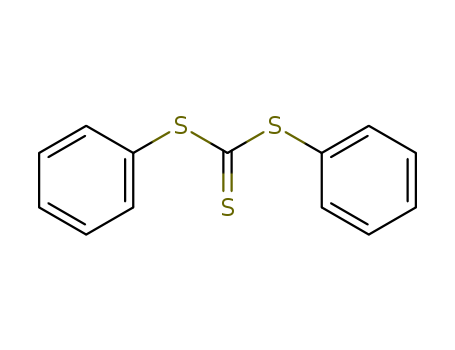
- Chemical Name:Diphenyl trithiocarbonate
- CAS No.:2314-54-7
- Molecular Formula:C13H10S3
- Molecular Weight:262.42
- Hs Code.:
- NSC Number:157932
- DSSTox Substance ID:DTXSID50945816
- Nikkaji Number:J142.393B
- Wikidata:Q82923264
- Mol file:2314-54-7.mol
Synonyms:Diphenyl trithiocarbonate;Phenyl trithiocarbonate;bis(phenylsulfanyl)methanethione;2314-54-7;Carbonotrithioic acid, diphenyl ester;Diphenyl carbonotrithioate;SCHEMBL3073488;DTXSID50945816;UHXNHMBEGXLRMF-UHFFFAOYSA-N;NSC157932;NSC-157932;Carbonic acid, trithio-, diphenyl ester;AB-131/40897085