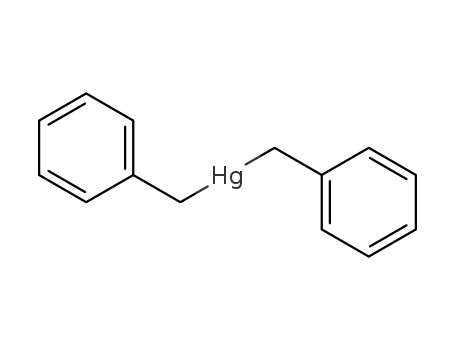
- Chemical Name:Dibenzylmercury
- CAS No.:780-24-5
- Molecular Formula:C14H14 Hg
- Molecular Weight:382.855
- Hs Code.:
- DSSTox Substance ID:DTXSID50228651
- Mol file:780-24-5.mol
Synonyms:Dibenzylmercury;780-24-5;Mercury,bis(phenylmethyl)-;MERCURY, DIBENZYL-;Mercury, bis(phenylmethyl)-;Mercury dibenzyl;BRN 3606128;AI3-19390;4-16-00-01706 (Beilstein Handbook Reference);Mercury, dibenzyl-,;DTXSID50228651;MFCD00014429;AKOS015839031;AS-58935;LS-89745;FT-0632951