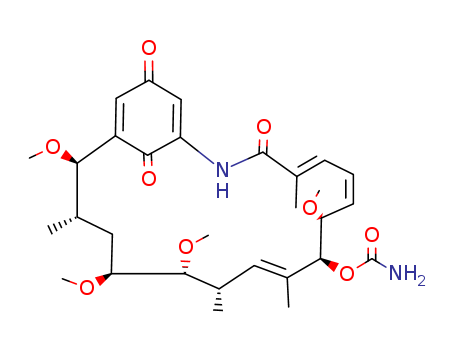
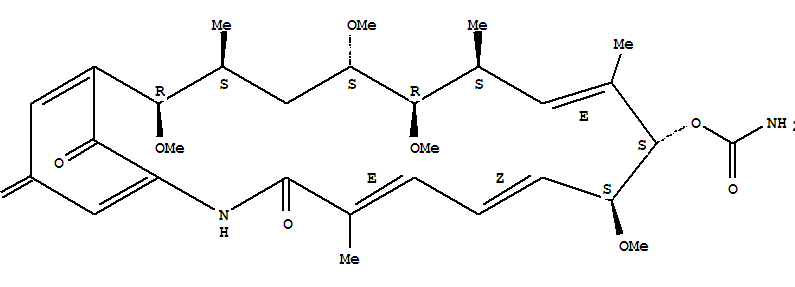
- Chemical Name:Herbimycin a
- CAS No.:70563-58-5
- Molecular Formula:C30H42N2O9
- Molecular Weight:574.671
- Hs Code.:29419000
- UNII:815WDV2HST
- Wikipedia:Herbimycin
- Wikidata:Q14200355
- NCI Thesaurus Code:C1124
- Metabolomics Workbench ID:53215
- ChEMBL ID:CHEMBL480499
- Mol file:70563-58-5.mol
Synonyms:geldanamycin, 17-demethoxy-15-methoxy-11-O-methyl-, (15R)-;herbimycin;herbimycin A;NSC 305978