- OXYGEN
- OXYGEN DIFLUORIDE
- Oxygen-fluorine acid
- 778-24-5Benzene,1,1'-(dimethylsilylene)bis-
- 7782-49-2Selenium
- 7782-50-5Chlorine
- 778-25-6Silanol,1-methyl-1,1-diphenyl-
- 7782-61-8Ferric nitrate nonahydrate
- 7782-63-0Ferrous sulfate heptahydrate
- 7782-64-1Manganese fluoride(MnF2)
- 7782-65-2Germane
- 7782-68-5Iodic acid (HIO3)
- 7782-75-4Phosphoric acid,magnesium salt (1:1), trihydrate (8CI,9CI)
Hot Products
- 7782-64-1Manganese(II) fluoride
- 7782-75-4MAGNESIUM HYDROGEN PHOSPHATE TRIHYDRATE
- 7782-77-6Nitrous acid
- 7782-78-7Nitrosylsulfuric acid
- 7782-82-3SODIUM HYDROGEN SELENITE
- 7783-46-2Lead fluoride
- 7783-48-4Strontium fluoride
- 7783-49-5Zinc fluoride
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
OXYGEN |
EINECS | 231-956-9 |
| CAS No. | 7782-44-7 | Density | 1.083 g/cm3 |
| PSA | 34.14000 | LogP | 0.06700 |
| Solubility | N/A | Melting Point |
-218 °C(lit.) |
| Formula | O2 | Boiling Point | -183 °C |
| Molecular Weight | 35.0226 | Flash Point | none |
| Transport Information | UN 1072 | Appearance | colourless gas |
| Safety | 17-45-36/37/39-26-61 | Risk Codes | 8-52/53-34 |
| Molecular Structure |
|
Hazard Symbols |
 O, O, C C
|
| Synonyms |
Molecular oxygen;Oxygen molecule; |
Article Data | 264 |
OXYGEN Synthetic route

B
- 7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| With perchloric acid; oxygen In acetonitrile Kinetics; | A 100% B n/a |
- 75-15-0
carbon disulfide

- 7722-84-1
dihydrogen peroxide

- 80937-33-3
oxygen

A
- 7446-09-5
sulfur dioxide

B
- 7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In gas Kinetics; byproducts: HS; other Radiation; H2O2 is photolysed at 266 nm (Nd:YAG laser) in a flow reactor, addn. of CS2 (NO) and O2 (N2); HO2 yield measurement by LMR (laser magnetic resonance), SO2 yield measurement by CIMS (chemical ionization mass spectometry); | A 90% B 95% |

| Conditions | Yield |
|---|---|
| With synthetic air; oxygen In gaseous matrix Kinetics; C2H4, CO and synthetic air mixture addn. into O3/O2 flow; | 66% |

| Conditions | Yield |
|---|---|
| With synthetic air; oxygen In gaseous matrix Kinetics; byproducts: hydroxyethylperoxy radical; C2H4 (1-3 ml/min) and synthetic air (3 ml/min) mixture addn. into O3/O2 flow; | A 20% B 39% |
- 3352-57-6
hydroxyl

- 14989-30-1
chlorine monoxide

A
- 7647-01-0
hydrogenchloride


C
- 80937-33-3
oxygen

D
- 7782-44-7
hydroperoxyl radical

| Conditions | Yield |
|---|---|
| In gas Kinetics; between 218 and 298 K, OH source: F + H2O or H + NO2, ClO source: Cl + O3, reaction carried out in a discharge-flow system; | A 9% B n/a C n/a D n/a |
| In gaseous matrix Kinetics; carrier gas: He; hydroxyl radicals were prepared by the reaction between H and NO2; ClO radicals were prepared by the reaction between O3 an Cl; resonance-fluorescence measurements; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; byproducts: C2H4, C2H5O2; Irradiation (UV/VIS); reaction of O2 with C2H5 radicals formed by flash-light photolysis (22 °C, 5-100 Torr total pressure); 99.9% of C2H5 reacted with formation of C2H5O2; rate constant;; | 0.1% |
| In neat (no solvent) Kinetics; byproducts: C2H4; reaction of C2H5 radicals with O2 at T<320 °C; mechanism;; |

| Conditions | Yield |
|---|---|
| In gas Kinetics; CH4-O2 reaction at 1100 °C and total pressure of 10 Torr; HO2 decay (1. order);; mass spectroscopy;; | |
| In neat (no solvent) CH4-O2 flames (12 Torr);; mass spectroscopy; assignment to HO2 is doubtful;; | |
| In neat (no solvent) CH4-O2 flames (70 Torr);; mass spectroscopy;; | |
| In neat (no solvent) byproducts: CH3, H2O2; reaction of 5 Torr CH4 with 3 Torr O2 at 1090 °C (reaction period 10E-2 s); collision yield; following reaction;; mass spectroscopy;; | 0.05% |
| In neat (no solvent) examination of the CH4-O2 reaction;; |

| Conditions | Yield |
|---|---|
| Kinetics; byproducts: OH, H2O, O2; at 298-386 K and 15-150 Torr; | |
| In neat (no solvent) Kinetics; byproducts: OH, H2O, O2; gaseous H2O2 was mixed with O+O2; rate constant;; | |
| In neat (no solvent) |

| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; reaction of OH (produced on reaction of H + NO2 -> OH + NO) and O3 (He carrier gas, 1.5 - 4 Torr, 300 K - 423 K); |

| Conditions | Yield |
|---|---|
| With water; acetic acid In water Kinetics; byproducts: O2; Irradiation (UV/VIS); | |
| In neat (no solvent) Kinetics; byproducts: O2; OH radicals formed from H+NO2 reacted with O3; rate constant;; UV absorption measurements;; |
OXYGEN Chemical Properties
.jpg)
IUPAC Name: Molecular oxygen
Canonical SMILES: O=O
InChI: InChI=1S/O2/c1-2
Molecular Formula: O2
Molecular Weight: 32.00
EINECS: 231-956-9
Classification Code: Gas, medicinal; Human Data; Mutation data; Reproductive Effect
Melting Point: -218 °C(lit.)
Stability: Stable. Vigorously supports combustion. Incompatible with phosphorus, organic materials, many powdered metals.
Index of Refraction: 1.122
Molar Refractivity: 2.36 cm3
Molar Volume: 29.5 cm3
Surface Tension: 20.2 dyne/cm
Density: 1.082 g/cm3
Enthalpy of Vaporization: 6.8 kJ/mol
Vapour Pressure of Oxygen (CAS NO.7782-44-7): 322000 mmHg at 25 °C
OXYGEN History
Oxygen (CAS NO.7782-44-7) was first discovered by Swedish pharmacist Carl Wilhelm Scheele. He had produced oxygen gas by heating mercuric oxide and various nitrates by about 1772. In 1891 Scottish chemist James Dewar was able to produce enough liquid oxygen to study. in 1895 by German engineer Carl von Linde and British engineer William Hampson. Later, in 1901, oxyacetylene welding was demonstrated for the first time by burning a mixture of acetylene and compressed O2. In 1923 the American scientist Robert H. Goddard became the first person to develop a rocket engine; the engine used gasoline for fuel and liquid oxygen as the oxidizer. Goddard successfully flew a small liquid-fueled rocket 56 m at 97 km/h on March 16, 1926 in Auburn, Massachusetts, USA.
OXYGEN Uses
Oxygen (CAS NO.7782-44-7) is used for primary metals manufacturing, chemicals manufacturing, oxidation processes, and partial oxidation processes. The steel industry prefers to use pure oxygen rather than air in processing iron. The oxygen reacts with elemental carbon to form carbon monoxide, which is processed with iron oxide so that carbon is incorporated into the iron metal, making it much lower melting and more pliable (fusible pig iron).
Oxygen is also used medically for patients who require mechanical ventilation, often at concentrations above 21% found in ambient air.
In other oxygen applications, metal fabrication involves cutting and welding with an oxygen-acetylene torch. Chemical manufacture use includes the formation of ethylene oxide, acrylic acid, propylene oxide, and vinyl acetate. Miscellaneous uses include sewage treatment, aeration, pulp and paper bleaching, and missile fuel.
OXYGEN Production
Oxygen (CAS NO.7782-44-7) is produced industrially by fractional distillation of liquefied air, use of zeolites to remove carbon dioxide and nitrogen from air, electrolysis of water and other means.
The manufacture of oxygen is described along with that of nitrogen (Fig. l) during the liquefaction of air. Pressure swing adsorption (Fig. 2) is also used to generate pure oxygen.
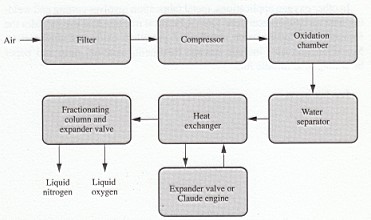
FIGURE 1 Manufacture of oxygen by the liquefaction of air.
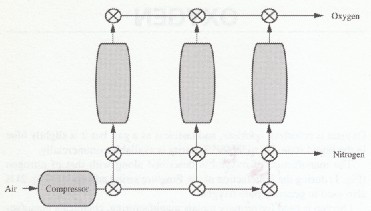
FIGURE 2 Pressure swing adsorption for oxygen generation and purification.
OXYGEN Toxicity Data With Reference
| 1. | cyt-ham:lng 80 pph | MUREAV Mutation Research. 57 (1978),27. | ||
| 2. | cyt-ham:lng 80 pph | ACATA5 Acta Anatomica. 94 (1976),520. | ||
| 3. | ihl-hmn TCLo:100 pph/14H:PUL | JAMAAP JAMA, Journal of the American Medical Association. 128 (1945),710. |
OXYGEN Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
OXYGEN Safety Profile
Human systemic effects by inhalation: cough and other pulmonary changes. Human teratogenic effects by inhalation: developmental abnormalities of the fetal cardiovascular system. Mutation data reported. Not toxic as gas. In liquid form it can cause severe “burns” and tissue damage on contact with the skin due to extreme cold.
Hazard Codes:  O,
O, C
C
Risk Statements: 8-52/53-34
R8 :Contact with combustible material may cause fire.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R34:Causes burns.
Safety Statements: 17-45-36/37/39-26-61
S17:Keep away from combustible material.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 1072 2.2
RTECS: RS2060000
F: 4.5-31
HazardClass: 2.2
OXYGEN Standards and Recommendations
DOT Classification: 2.2; Label: Nonflammable Gas, Oxidizer
OXYGEN Analytical Methods
For occupational chemical analysis use NIOSH: Oxygen (field-readable) 6601.
OXYGEN Specification
Oxygen (CAS NO.7782-44-7), its Synonyms are Hyperoxia ; LOX ; Liquid oxygen ; Molecular oxygen ; Oxigeno ; Oxigeno [Spanish] ; Oxygen ; Oxygen molecule ; Oxygen, liquified ; Oxygen-16 ; Oxygene ; Oxygene [French] ; Oxygenium ; Oxygenium medicinale ; Pure oxygen ; Sauerstoff .

