Krypton
Krypton General
| Name:Krypton | Symbol:Kr |
| Type:Noble Gas | Atomic weight:83.80 |
| Density @ 293 K: 0.003708 g/cm3 | Atomic volume:38.9 cm3/mol |
|
Discovered:
William Ramsay and Morris Travers discovered krypton in 1898. They discovered it in the residue remaining after liquid air had been fractionally distilled. With the oxygen and nitrogen gone, a bright yellow spectral line that was neither sodium nor helium revealed the presence of a new element. The element name comes from the Greek word 'kryptos', meaning hidden. |
|
Krypton States
| State (s, l, g):gas | |
| Melting point:115.9 K (-157.3 °C) | Boiling point:119.4 K (-153.2 °C) |
Krypton Energies
| Specific heat capacity:0.248 J g-1 K-1 | Heat of atomization:0 kJ mol-1 |
| Heat of fusion: 1.638 kJ mol-1 | Heat of vaporization :9.029 kJ mol-1 |
| 1st ionization energy:1350.7 kJ mol-1 | 2nd ionization energy:2350.3 kJ mol-1 |
| 3rd ionization energy:3565.1 kJ mol-1 | Electron affinity:kJ mol-1 |
Krypton Oxidation & Electrons
| Shells:2,8,18,8 | Electron configuration:[Ar] 3d10 4s2 4p6 |
| Minimum oxidation number:0 | Maximum oxidation number:2 |
| Min. common oxidation no.:0 | Max. common oxidation no.:2 |
| Electronegativity (Pauling Scale): 3 | Polarizability volume:2.5 Å3 |
Krypton Appearance & Characteristics
| Structure:fcc: face-centered cubic | Color:Colorless |
| Hardness:mohs | |
|
Harmful effects:
Krypton is considered to be non-toxic. |
|
|
Characteristics:
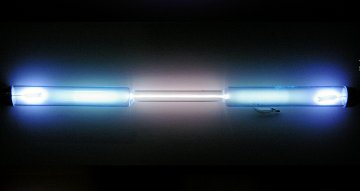
Krypton is a colorless, odorless, inert gas. Uses: Krypton is used in lighting products. Ionized krypton gas appears whitish - see photo on left - which makes krypton-based bulbs useful as a brilliant white light source in high speed photography. An important lighting use is also in high-powered, flashing airport runway lights. |
|
Krypton Reactions
| Reaction with air:none | Reaction with 6 M HCl:none |
| Reaction with 15 M HNO3:none | Reaction with 6 M NaOH:none |
Krypton Compounds
| Oxide(s):none | Chloride(s):none |
| Hydride(s):none |
Krypton Radius
| Atomic radius:88 pm | Ionic radius (1+ ion):pm |
| Ionic radius (2+ ion):pm | Ionic radius (3+ ion):pm |
| Ionic radius (2- ion):pm | Ionic radius (1- ion):pm |
Krypton Conductivity
| Thermal conductivity:0.01 W m-1 K-1 | Electrical conductivity:S cm-1 |
Krypton Abundance & Isotopes
| Abundance earth's crust: 100 parts per trillion by weight, 30 parts per trillion by moles | |
| Abundance solar system:parts per million by weight, parts per million by moles | |
| Cost, pure:$33 per 100g | |
| Cost, bulk:$ per 100g | |
|
Source:
Krypton is obtained commercially by fractional distillation of liquid air. |
|
|
Isotopes:
Krypton has 25 isotopes whose half-lives are known, with mass numbers 71 to 95. Of these, six are stable: 78Kr, 80Kr, 82Kr, 83Kr, 84Kr and 86Kr. The most abundant isotope is 84Kr at 57.03%. |
|
Krypton Other
|
Other:
|
|
Prev: Bromine Next: Rubidium |