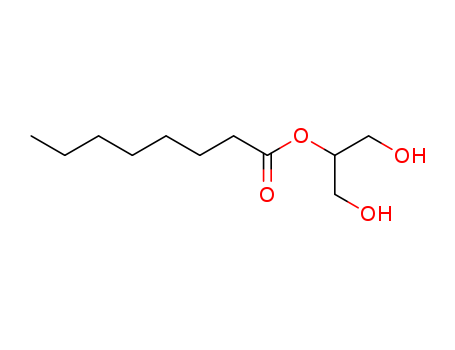
- Chemical Name:Glyceryl 2-caprylate
- CAS No.:4228-48-2
- Molecular Formula:C11H22O4
- Molecular Weight:218.293
- Hs Code.:
- UNII:O8TCW8DUW8
- DSSTox Substance ID:DTXSID20195104
- Nikkaji Number:J118.317F
- Wikidata:Q27285489
- Mol file:4228-48-2.mol
Synonyms:Glyceryl 2-caprylate;beta-Monocaprylin;4228-48-2;Octanoin, 2-mono-;1,3-dihydroxypropan-2-yl Octanoate;Octanoic acid 2-monoglyceride;Octanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester;UNII-O8TCW8DUW8;O8TCW8DUW8;2-monocapryloylglycerol;.BETA.-MONOCAPRYLIN;SCHEMBL239865;DTXSID20195104;Q27285489