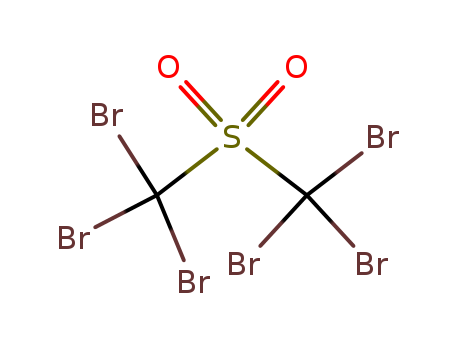
- Chemical Name:Sulphonylbis(tribromomethane)
- CAS No.:7241-13-6
- Molecular Formula:C6H13N2+
- Molecular Weight:567.511
- Hs Code.:
- European Community (EC) Number:230-642-9
- DSSTox Substance ID:DTXSID60222706
- Nikkaji Number:J56.073A
- Wikidata:Q83101023
- Mol file:7241-13-6.mol
Synonyms:Sulphonylbis(tribromomethane);7241-13-6;SULPHONYLBIS[TRIBROMOMETHANE];EINECS 230-642-9;bis(tribromomethyl) sulfone;C2Br6O2S;Bis-(tribrommethyl)-sulfon;SCHEMBL121999;Methane, sulfonylbis[tribromo-;C2-Br6-O2-S;DTXSID60222706