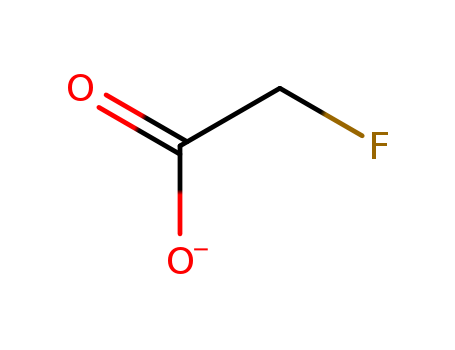
- Chemical Name:Fluoroacetate
- CAS No.:513-62-2
- Molecular Formula:C2H2FO2-
- Molecular Weight:77.0351
- Hs Code.:
- DSSTox Substance ID:DTXSID10199297
- Nikkaji Number:J1.706.888A
- Wikidata:Q27102870
- Metabolomics Workbench ID:191261
- Mol file:513-62-2.mol
Synonyms:Fluoroacetate;513-62-2;FCH2CO2 anion;Acetic acid, fluoro-, ion(1-);2-fluoroacetate;Cymoric acid;Alpha-fluoroacetic acid;fluoroacetic acid, ion(1-);C2H2FO2;CHEBI:18172;DTXSID10199297;NCGC00178188-01;Q27102870