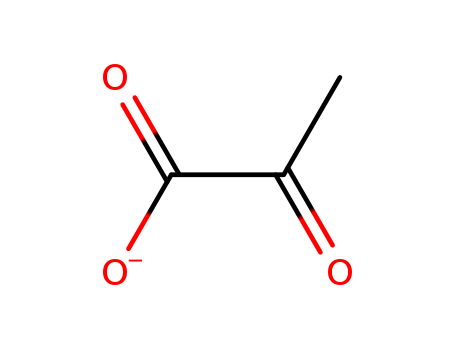
- Chemical Name:Pyruvate
- CAS No.:57-60-3
- Molecular Formula:C3H3O3-
- Molecular Weight:87.055
- Hs Code.:
- UNII:HO43T60JMG
- DSSTox Substance ID:DTXSID50205604
- Nikkaji Number:J469.720K
- Wikidata:Q27089397
- NCI Thesaurus Code:C116012
- Mol file:57-60-3.mol
Synonyms:Acid, Pyruvic;Pyruvate;Pyruvic Acid