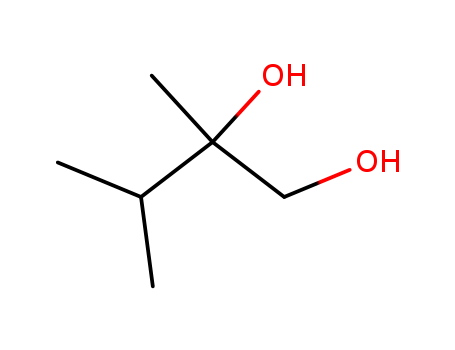
- Chemical Name:2,3-Dimethylbutane-1,2-diol
- CAS No.:66553-15-9
- Molecular Formula:C6H14O2
- Molecular Weight:118.176
- Hs Code.:2905399090
- European Community (EC) Number:266-403-0
- NSC Number:126861
- DSSTox Substance ID:DTXSID801310266
- Nikkaji Number:J310.115K
- Mol file:66553-15-9.mol
Synonyms:2,3-Dimethylbutane-1,2-diol;66553-15-9;2,3-dimethyl-1,2-butanediol;EINECS 266-403-0;1,2-Butanediol,2,3-dimethyl-;NSC126861;SCHEMBL271545;1,2-Butanediol, 2,3-dimethyl-;DTXSID801310266;AKOS019065127;NSC 126861;NSC-126861;EN300-1240420