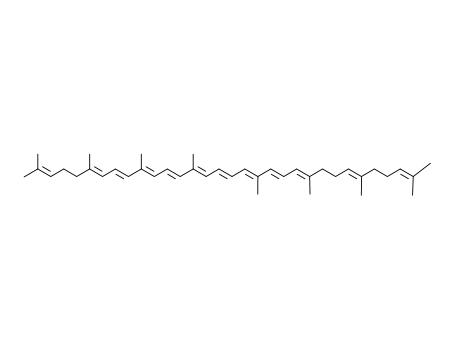
Multi-step reaction with 9 steps
1: 40 percent / KOH / tetrahydrofuran / 2 h / Ambient temperature
2: 76 percent / NaBH4 / diethyl ether; methanol / 0.25 h
3: PBr3 / diethyl ether / 2 h / 1.) Et2O, 0 deg C; 2.) room temperature, 2 h
4: 2 h / 60 °C
5: 40 percent / KOH / tetrahydrofuran / 1 h / Ambient temperature
6: 1.) DIBAH; 2.) MnO2 / 1.) THF, 0 deg C, 15 min; 2.) Et2O, room temperature, 30 min
7: 43 percent / KOH / tetrahydrofuran / 1 h / 0 °C
8: 1.) DIBAH; 2.) MnO2 / 1.) THF, 0 deg C, 15 min; 2.) Et2O, room temperature, 30 min
9: 6 percent / NaOMe / methanol; dimethylformamide / 3 h / Ambient temperature
With
manganese(IV) oxide; potassium hydroxide; sodium tetrahydroborate; sodium methylate; phosphorus tribromide; diisobutylaluminium hydride;
In
tetrahydrofuran; methanol; diethyl ether; N,N-dimethyl-formamide;
DOI:10.1002/hlca.19850680604