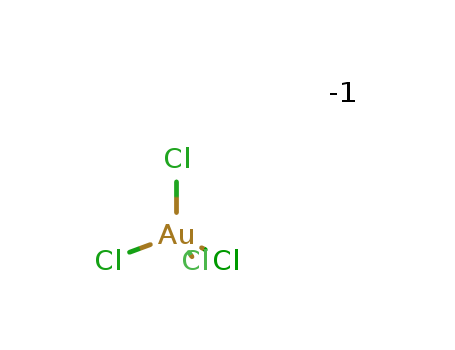
- Chemical Name:Tetrachloroaurate
- CAS No.:14337-12-3
- Molecular Formula:AuCl4
- Molecular Weight:338.779
- Hs Code.:
- DSSTox Substance ID:DTXSID601014503
- Nikkaji Number:J276.358C
- Mol file:14337-12-3.mol
Synonyms:Tetrachloroaurate;14337-12-3;Aurate(1-), tetrachloro-, (SP-4-1)-;gold(3+);tetrachloride;Aurate(1-), tetrachloro-;AuCl4;Au-Cl4;Tetrachloroaurate (III);DTXSID601014503