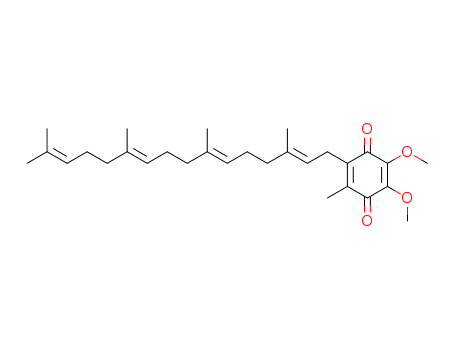
- Chemical Name:Coenzyme Q4
- CAS No.:4370-62-1
- Molecular Formula:C29H42 O4
- Molecular Weight:454.65
- Hs Code.:
- NSC Number:266773
- UNII:I7G555QPVU
- DSSTox Substance ID:DTXSID901345979
- Nikkaji Number:J93.224H
- Metabolomics Workbench ID:29078
- ChEMBL ID:CHEMBL407116
- Mol file:4370-62-1.mol
Synonyms:COENZYME Q4;4370-62-1;Ubiquinone-4;Ubiquinone 4;Ubiquinone Q4;CoQ4;I7G555QPVU;2,3-dimethoxy-5-methyl-6-[(2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl]cyclohexa-2,5-diene-1,4-dione;2,3-Dimethoxy-5-methyl-6-(geranylgeranyl)-1,4-benzoquinone;NSC-266773;Ubiquinone-20;2,3-dimethoxy-5-methyl-6-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl)cyclohexa-2,5-diene-1,4-dione;NSC266773;Ubidihydroquinone Q4;COENZYMEQ4;UNII-I7G555QPVU;CHEMBL407116;SCHEMBL6279406;CHEBI:149479;XGCJRRDNIMSYNC-INVBOZNNSA-N;DTXSID901345979;6-Methyl-3-(1-methylpropyl)uracil;LMPR02010003;NSC 266773;C6F752F6-69B0-4CC2-9B43-B8E7D1701247;2,3-dimethoxy-5-methyl-6-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-2,5-Cyclohexadiene-1,4-dione;2,3-dimethoxy-5-methyl-6-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-p-Benzoquinone;2,3-dimethoxy-5-methyl-6-[(2E,6E,10E)-3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl]-2,5-Cyclohexadiene-1,4-dione;2,5-CYCLOHEXADIENE-1,4-DIONE, 2,3-DIMETHOXY-5-METHYL-6-((2E,6E,10E)-3,7,11,15-TETRAMETHYL-2,6,10,14-HEXADECATETRAEN-1-YL)-;p-Benzoquinone, 2,3-dimethoxy-5-methyl-6-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-