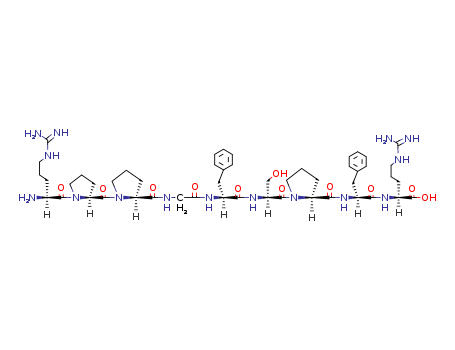
- Chemical Name:Bradykinin
- CAS No.:58-82-2
- Molecular Formula:C50H73 N15 O11
- Molecular Weight:1060.22
- Hs Code.:
- Mol file:58-82-2.mol
Synonyms:Arginine,N2-[N-[1-[N-[N-[N-[1-(1-arginyl-L-prolyl)-L-prolyl]glycyl]-3-phenylalanyl]seryl]prolyl]-3-phenylalanyl]-(6CI); 1020: PN: EP2071336 PAGE: 73 unclaimed sequence; 1021: PN: WO2006091861SEQID: 1021 claimed protein; 107: PN: WO2006069765 SEQID: 107 claimed protein;10: PN: WO2008008766 SEQID: 124 unclaimed sequence; 111: PN: WO2008058016SEQID: 112 claimed protein; 115: PN: WO2008104000 TABLE: 4 unclaimed sequence;11: PN: US20040209344 SEQID: 7 unclaimed protein; 13: PN: WO0023092 SEQID: 13unclaimed protein; 147: PN: US20030119021 SEQID: 25 unclaimed protein; 14: PN:US20090088553 SEQID: 88 unclaimed protein; 14: PN: US6525021 SEQID: 13unclaimed protein; 153: PN: WO2006067198 SEQID: 165 claimed protein; 156: PN:WO2009126292 TABLE: 4 unclaimed sequence; 15: PN: US6258776 SEQID: 24 unclaimedprotein; 15: PN: WO2007058336 SEQID: 15 claimed sequence; 165: PN:US20050175581 SEQID: 165 unclaimed protein; 16: PN: WO2006079099 SEQID: 1unclaimed protein; 175: PN: WO2008008805 SEQID: 178 unclaimed protein; 187: PN:US20030176421 PAGE: 54-55 claimed protein; 18: PN: JP2006280210 SEQID: 18unclaimed protein; 19: PN: US20060223140 SEQID: 18 claimed protein; 19: PN:WO2007022248 SEQID: 19 claimed protein; 1: PN: CN101344527 PAGE: 6 claimedsequence; 1: PN: JP2005049164 SEQID: 1 claimed protein; 1: PN: US20040198666SEQID: 1 unclaimed protein; 1: PN: US20040220110 PAGE: 2 unclaimed protein; 1:PN: US20070015715 SEQID: 1 unclaimed sequence; 1: PN: US7605120 SEQID: 1unclaimed sequence; 1: PN: WO02102835 SEQID: 3 unclaimed protein; 1: PN:WO2006130718 SEQID: 1 unclaimed protein; 1: PN: WO2006134125 SEQID: 22 claimedprotein; 227: PN: WO2009118205 PAGE: 176 unclaimed sequence; 229: PN:US20090048431 SEQID: 227 claimed sequence; 24: PN: US20080226503 TABLE: 1unclaimed sequence; 2: PN: WO2005042027 SEQID: 1 unclaimed protein; 2: PN:WO2005057221 PAGE: 17 unclaimed protein; 360: PN: WO2009117524 SEQID: 5unclaimed sequence; 38: PN: WO2006017688 SEQID: 38 unclaimed protein;