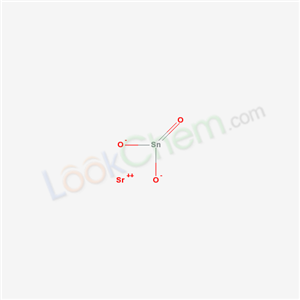
Base Information
Edit
- Chemical Name:STRONTIUM STANNATE
- CAS No.:12143-34-9
- Molecular Formula:O3SnSr
- Molecular Weight:254.33
- Hs Code.:
- Mol file:12143-34-9.mol
Synonyms:Sulfuric acid,compounds,strontium salt (1:1);Strontium tin trioxide;dioxido-oxo-tin; strontium(+2) cation;Strontium stannate;