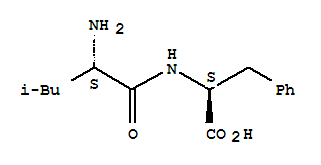
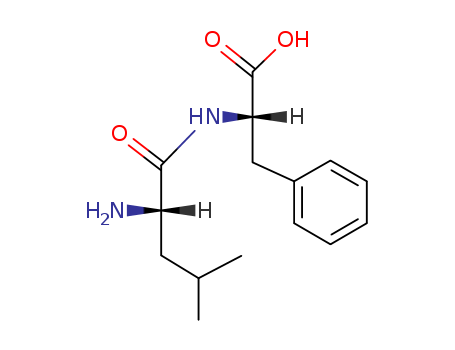
- Chemical Name:Leu-phe
- CAS No.:3063-05-6
- Molecular Formula:C15H22 N2 O3
- Molecular Weight:278.351
- Hs Code.:
- DSSTox Substance ID:DTXSID501036415
- Nikkaji Number:J80.629C
- Wikidata:Q27141902
- Metabolomics Workbench ID:78869
- ChEMBL ID:CHEMBL54936
- Mol file:3063-05-6.mol
Synonyms:(2S,3S)-(2-2H,3-2H)-leucine-(S)-phenylalanine;Leu-Phe;leucyl-phenylalanine;leucylphenylalanine