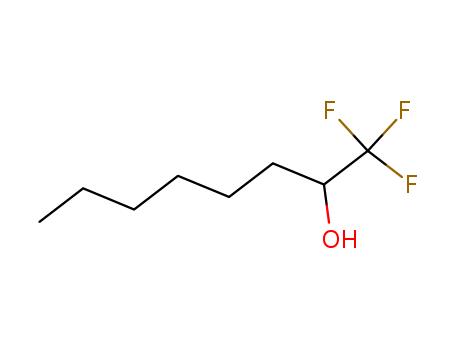
- Chemical Name:1,1,1-Trifluoro-2-octanol
- CAS No.:453-43-0
- Deprecated CAS:128298-65-7
- Molecular Formula:C8H15 F3 O
- Molecular Weight:184.202
- Hs Code.:2905590090
- European Community (EC) Number:207-220-8
- NSC Number:42759
- DSSTox Substance ID:DTXSID00883239
- Nikkaji Number:J122.147G
- Mol file:453-43-0.mol
Synonyms:1,1,1-Trifluoro-2-octanol;1,1,1-Trifluorooctan-2-ol;453-43-0;2-Octanol, 1,1,1-trifluoro-;EINECS 207-220-8;NSC 42759;C8H15F3O;1-(Trifluoromethyl)heptan-1-ol;NSC42759;1-trifluoromethyl-heptanol;1,1-Trifluoro-2-octanol;2-Octanol,1,1-trifluoro-;SCHEMBL574572;DTXSID00883239;1,1,1-trifluoro-2-octyl alcohol;C8-H15-F3-O;MFCD00069163;NSC-42759;AKOS009159476;SB47078;FT-0605190;FT-0733901;FT-0773829;E87067