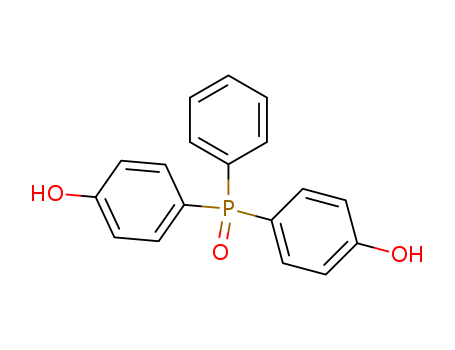
- Chemical Name:BIS(4-HYDROXYPHENYL)PHENYLPHOSPHINE OXIDE
- CAS No.:795-43-7
- Molecular Formula:C18H15O3P
- Molecular Weight:310.289
- Hs Code.:
- European Community (EC) Number:212-347-7
- DSSTox Substance ID:DTXSID00229719
- Nikkaji Number:J193.182B
- Wikidata:Q72446055
- Mol file:795-43-7.mol
Synonyms:4-[(4-Hydroxyphenyl)-phenyl-phosphoryl]phenol;4,4'-(Phenylphosphoryl)diphenol;Bis(4-hydroxyphenyl)phenylphosphine oxide;