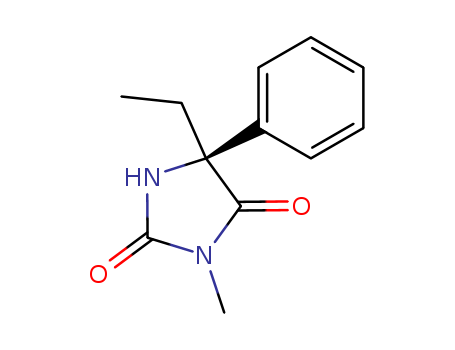
- Chemical Name:(S)-Mephenytoin
- CAS No.:70989-04-7
- Molecular Formula:C12H14 N2 O2
- Molecular Weight:218.255
- Hs Code.:29332100
- European Community (EC) Number:621-693-1
- UNII:D9818430MW
- DSSTox Substance ID:DTXSID7046126
- Nikkaji Number:J428.022I
- Wikidata:Q27276264
- Metabolomics Workbench ID:144178
- ChEMBL ID:CHEMBL1743264
- Mol file:70989-04-7.mol
Synonyms:(S)-Mephenytoin;70989-04-7;S-Mephenytoin;(S)-(+)-Mephenytoin;(5s)-5-ethyl-3-methyl-5-phenylimidazolidine-2,4-dione;Mephenytoin, (+)-;(S)-5-ethyl-3-methyl-5-phenylimidazolidine-2,4-dione;Mephenytoin, D-;(+)-Mephenytoin;(+)-Mesantoin;(+)-S-Mephenytoin;d-Mephenytoin;(5S)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione;DTXSID7046126;UNII-D9818430MW;D9818430MW;NCGC00160394-01;DTXCID5026126;CAS-70989-04-7;MFCD00270025;(S)-5-Ethyl-3-methyl-5-phenylhydantoin;MEPHENYTOIN, (S)-;SCHEMBL21768;CHEMBL1743264;BDBM21361;HY-B1184A;(S)-(+)-Mephenytoin, 98%;C(Nc1ccc(Cl)cc1)c2ccc(C)cc2;CHEBI:179062;EX-A6720;Tox21_111783;Tox21_111783_1;NCGC00160394-02;NCGC00165924-04;CS-0102758;A13366;C20130;S10442;(+)-5-ETHYL-3-METHYL-5-PHENYLHYDANTOIN;(S)-(+)-Mephenytoin, solid, >=98% (HPLC);A934834;EN300-10845170;Q27276264;2,4-Imidazolidinedione, 5-ethyl-3-methyl-5-phenyl-, (5S)-;2,4-Imidazolidinedione, 5-ethyl-3-methyl-5-phenyl-, (S)-


 Xn
Xn


