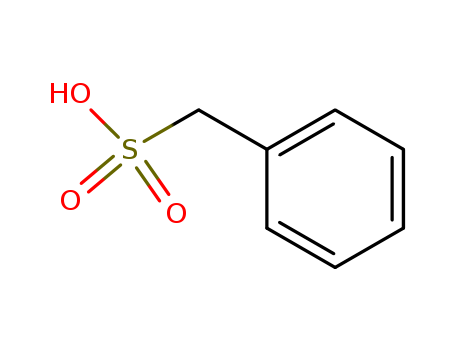
- Chemical Name:Phenylmethanesulfonic acid
- CAS No.:100-87-8
- Molecular Formula:C7H8 O3 S
- Molecular Weight:172.205
- Hs Code.:2904100000
- European Community (EC) Number:202-897-6,853-857-5
- UNII:5BRP81V98H
- DSSTox Substance ID:DTXSID2059221
- Nikkaji Number:J182.139C
- Wikidata:Q27261795
- Metabolomics Workbench ID:147824
- ChEMBL ID:CHEMBL1171433
- Mol file:100-87-8.mol
Synonyms:benzylsulfonic acid;phenylmethanesulfonic acid;phenylmethanesulfonic acid, sodium salt