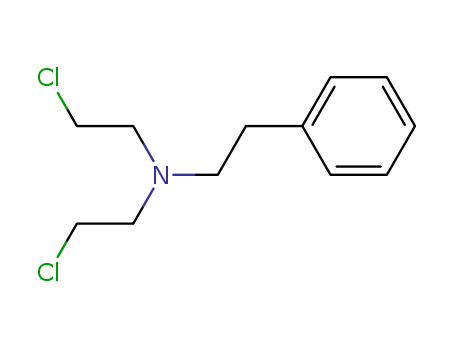
- Chemical Name:Phenethylamine, N,N-bis(2-chloroethyl)-
- CAS No.:1138-79-0
- Molecular Formula:C12H17 Cl2 N
- Molecular Weight:246.18
- Hs Code.:
- NSC Number:108713
- DSSTox Substance ID:DTXSID60150555
- Nikkaji Number:J71.119E
- Wikidata:Q83016680
- Mol file:1138-79-0.mol
Synonyms:1138-79-0;N,N-Bis(2-chloroethyl)benzeneethanamine;N,N-Bis(2-chloroethyl)phenethylamine;N-Phenethyl-N,N-di-2-chloroethylamine;Phenethylamine, N,N-bis(2-chloroethyl)-;NSC 108713;BRN 2724152;Benzeneethanamine, N,N-bis(2-chloroethyl)-;Triethylamine, 2,2'-dichloro-2''-phenyl-;NSC108713;WLN: G2N2G2R;4-12-00-02455 (Beilstein Handbook Reference);SCHEMBL4269278;DTXSID60150555;Phenethylamine,N-bis(2-chloroethyl)-;NSC-108713;Triethylamine,2'-dichloro-2''-phenyl-;Benzeneethanamine,N-bis(2-chloroethyl)-