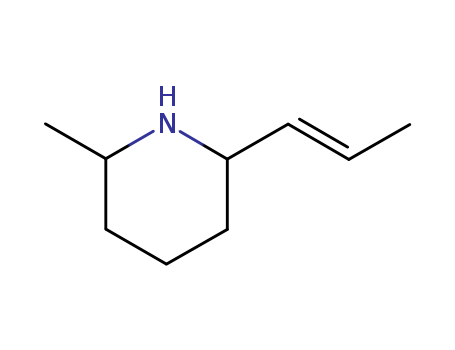
- Chemical Name:(2R,6R)-2-methyl-6-prop-1-enyl-piperidine
- CAS No.:501-02-0
- Molecular Formula:C9H17N
- Molecular Weight:139.241
- Hs Code.:
- Mol file:501-02-0.mol
Synonyms:Pinidine(6CI,7CI,8CI); Piperidine, 2-methyl-6-(1-propenyl)-, [2R-[2a,6a(E)]]-; Piperidine, 2-methyl-6-(1E)-1-propenyl-,(2R,6R)- (9CI); (-)-Pinidine; cis-(-)-6-Propenyl-2-pipecoline