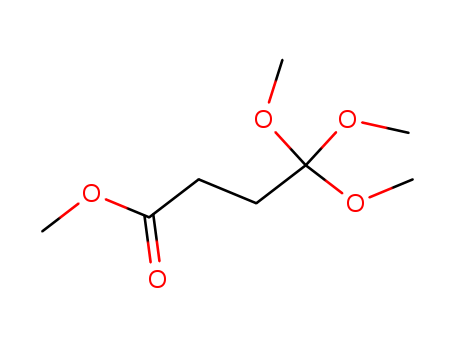
- Chemical Name:4,4,4-TriMethoxybutanoic Acid Methyl Ester
- CAS No.:71235-00-2
- Molecular Formula:C8H16O5
- Molecular Weight:192.21000
- Hs Code.:
- Mol file:71235-00-2.mol
Synonyms:trimethyl 3-(methoxycarbonyl)orthopropionate;Butanoic acid,4,4,4-trimethoxy-,methyl ester;methyl 4,4,4-trimethoxy butanoate;monoorthosuccinic acid tetramethyl ester;Monoorthobernsteinsaeure-tetramethylester;