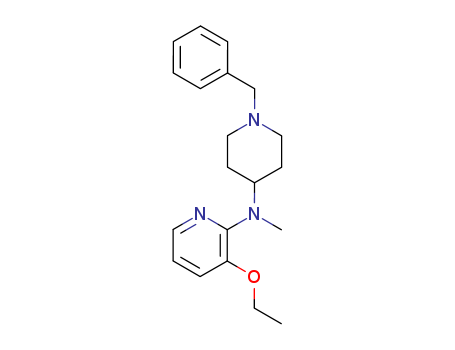
- Chemical Name:3-Isopropoxy-N-methyl-N-(1-(phenylmethyl)-4-piperidinyl)-2-pyridinylamine
- CAS No.:170856-57-2
- Molecular Formula:C20H27 N3 O
- Molecular Weight:339.4745
- Hs Code.:
- DSSTox Substance ID:DTXSID40168956
- Nikkaji Number:J829.152G
- Wikidata:Q27089043
- Pharos Ligand ID:AWLXD52H3XKL
- ChEMBL ID:CHEMBL1179189
- Mol file:170856-57-2.mol
Synonyms:3-isopropoxy-N-methyl-N-(1-(phenylmethyl)-4-piperidinyl)-2-pyridinylamine;PNU 101958;U 101958;U-101958