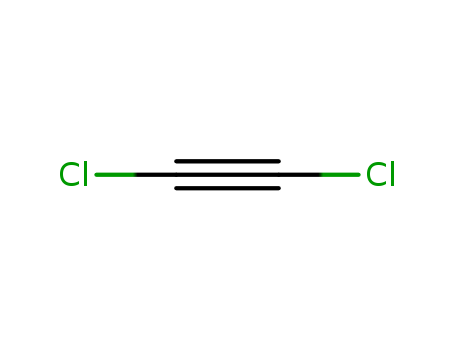
Chemical Property of Dichloroacetylene
Edit
Chemical Property:
- Vapor Pressure:122mmHg at 25°C
- Melting Point:-1.8oC
- Refractive Index:1.4279
- Boiling Point:74.1°Cat760mmHg
- Flash Point:7.3°C
- PSA:0.00000
- Density:1.403g/cm3
- LogP:1.38240
- XLogP3:2.3
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:0
- Rotatable Bond Count:0
- Exact Mass:93.9377054
- Heavy Atom Count:4
- Complexity:46.9
- Purity/Quality:
-
98%,99%, *data from raw suppliers
Safty Information:
- Pictogram(s):
Confirmed carcinogen. TLV: ceiling 0.1 ppm.
- Hazard Codes:E,Xn
- Statements:
2-40-48/20
- Safety Statements:
36/37
- MSDS Files:
-
SDS file from LookChem
Useful:
- Chemical Classes:Other Classes -> Halogenated Aliphatics, Unsaturated
- Canonical SMILES:C(#CCl)Cl
- Inhalation Risk:A harmful contamination of the air can be reached very quickly on evaporation of this substance at 20 °C.
- Effects of Short Term Exposure:The substance may cause effects on the nervous system and kidneys. This may result in tissue lesions, impaired functions and kidney impairment.
-
Uses
DCA is not commercially available in large quantities. It is
reportedly a by-product of the synthesis of vinylidene chloride
and is not known to be used commercially. By-product in synthesis of
vinylidene chloride; decomposition product of
trichloroethylene under alkaline conditions