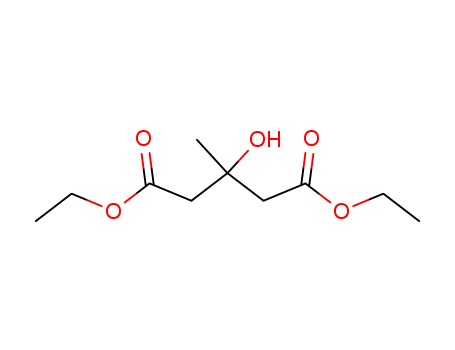
- Chemical Name:Diethyl 3-hydroxy-3-methylglutarate
- CAS No.:73489-84-6
- Molecular Formula:C10H18O5
- Molecular Weight:218.25
- Hs Code.:29171900
- European Community (EC) Number:277-481-0
- DSSTox Substance ID:DTXSID30994246
- Nikkaji Number:J295.229G
- Wikidata:Q82985107
- ChEMBL ID:CHEMBL156298
- Mol file:73489-84-6.mol
Synonyms:Diethyl 3-hydroxy-3-methylglutarate;73489-84-6;diethyl 3-hydroxy-3-methylpentanedioate;EINECS 277-481-0;CHEMBL156298;DTXSID30994246;FT-0718710;3-Hydroxy-3-methylpentanedioic acid diethyl ester