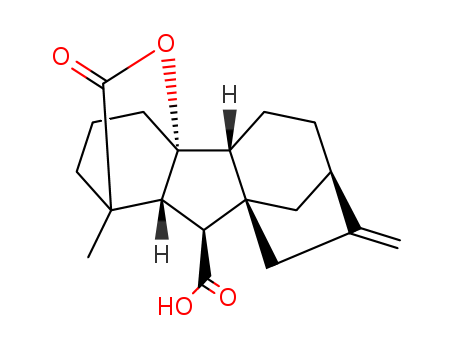
- Chemical Name:Gibberellin A9
- CAS No.:427-77-0
- Molecular Formula:C19H24O4
- Molecular Weight:316.397
- Hs Code.:
- UNII:SWC5WIK98K
- Nikkaji Number:J4.735J
- Wikidata:Q27110172
- Mol file:427-77-0.mol
Synonyms:Gibberellin A9;427-77-0;SWC5WIK98K;UNII-SWC5WIK98K;Gibbane-1,10-dicarboxylic acid, 4a-hydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1alpha,4aalpha,4bbeta,10beta)-;(1R,2R,5R,8R,9S,10R,11R)-11-Methyl-6-methylidene-16-oxo-15-oxapentacyclo[9.3.2.15,8.01,10.02,8]heptadecane-9-carboxylic acid;(1R,4aR,4bR,7R,9aR,10S,10aR)-1-methyl-8-methylene-13-oxododecahydro-4a,1-(epoxymethano)-7,9a-methanobenzo[a]azulene-10-carboxylic acid;Gibbane-1,10-dicarboxylic acid, 4a-hydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1.alpha.,4a.alpha.,4b.beta.,10.beta.)-;GA9;SCHEMBL3370009;CHEBI:29605;Q27110172;(1R,2R,5R,8R,9S,10R,11R)-11-methyl-6-methylidene-16-oxo-15-oxapentacyclo[9.3.2.1(5,8).0(1,10).0(2,8)]heptadecane-9-carboxylic acid;1beta-methyl-8-methylidene-13-oxo-4a,1alpha-epoxymethano-4aalpha,4bbeta-gibbane-10beta-carboxylic acid;1H-7,9A-METHANOBENZ(A)AZULENE-1,10-DICARBOXYLIC ACID, DODECAHYDRO-4A-HYDROXY-1-METHYL-8-METHYLENE-, 1,4A-LACTONE;4A.ALPHA.,4B.BETA.-GIBBANE-1.ALPHA.,10.BETA.-DICARBOXYLIC ACID, 4A-HYDROXY-1-METHYL-8-METHYLENE-, 1,4A-LACTONE