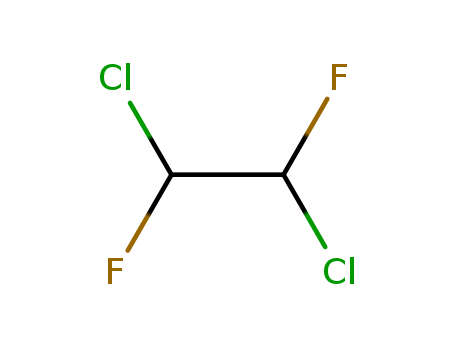
- Chemical Name:1,2-Dichloro-1,2-difluoroethane
- CAS No.:431-06-1
- Molecular Formula:C2H2 Cl2 F2
- Molecular Weight:134.941
- Hs Code.:2903791090
- European Community (EC) Number:207-070-3
- DSSTox Substance ID:DTXSID70861928
- Nikkaji Number:J85.962A
- Mol file:431-06-1.mol
Synonyms:1,2-Dichloro-1,2-difluoroethane;431-06-1;C2H2Cl2F2;EINECS 207-070-3;HCFC-132;Ethane, 1,2-dichloro-1,2-difluoro-;SCHEMBL201983;1,2-difluor-1,2-dichlorethan;DTXSID70861928;1,2-Difluoro-1,2-dichloroethane;1,2-dichloro-1,2-difluoro-ethane;MFCD00045245;AKOS006227825;LS-185335;FT-0606373;1,2-bis(chloranyl)-1,2-bis(fluoranyl)ethane;A826156