Base Information
Edit
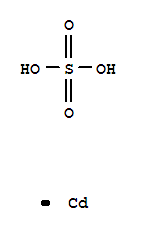
- Chemical Name:Cadmium sulfate
- CAS No.:10124-36-4
- Deprecated CAS:31119-53-6,62642-07-3,62642-07-3
- Molecular Formula:CdSO4
- Molecular Weight:208.46
- Hs Code.:28332990
- European Community (EC) Number:233-331-6
- ICSC Number:1318
- UN Number:2570
- UNII:947UNF3Z6O
- DSSTox Substance ID:DTXSID1020229
- Nikkaji Number:J3.117H
- Wikipedia:Cadmium sulfate
- Wikidata:Q414806
- NCI Thesaurus Code:C45895
- Mol file:10124-36-4.mol
Synonyms:Cadmiummonosulfate;Cadmium sulfate (Cd(SO4));Cadmium sulforicum;


 T+;
T+;  N
N
