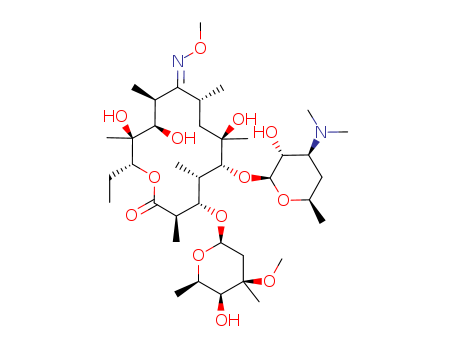
Suppliers and Price of Lexithromycin
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- ChemScene
- Lexithromycin 98.80%
- 25mg
- $ 550.00
- ChemScene
- Lexithromycin 98.80%
- 5mg
- $ 150.00
- ChemScene
- Lexithromycin 98.80%
- 100mg
- $ 1650.00
- Cayman Chemical
- Lexithromycin ≥98%
- 25mg
- $ 677.00
- Cayman Chemical
- Lexithromycin ≥98%
- 5mg
- $ 151.00
- American Custom Chemicals Corporation
- LEXITHROMYCIN 95.00%
- 5MG
- $ 502.99
-
Total 8 raw suppliers