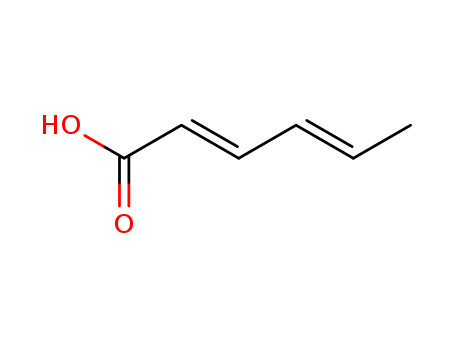
- Chemical Name:Sorbic Acid
- CAS No.:110-44-1
- Deprecated CAS:91751-55-2,1197240-56-4,22500-92-1
- Molecular Formula:C6H8O2
- Molecular Weight:112.128
- Hs Code.:29161930
- European Community (EC) Number:203-768-7,640-983-9
- ICSC Number:1284
- UNII:X045WJ989B
- DSSTox Substance ID:DTXSID3021277
- Nikkaji Number:J394.115I,J628.002A,J2.449J
- Wikipedia:Sorbic_acid
- Wikidata:Q407131
- NCI Thesaurus Code:C82293
- RXCUI:1311139
- Metabolomics Workbench ID:533
- ChEMBL ID:CHEMBL250212
- Mol file:110-44-1.mol
Synonyms:Acid, Hexadienoic;Acid, Propenylacrylic;Acid, Sorbic;Hexadienoic Acid;Potassium Sorbate;Propenylacrylic Acid;Sodium Sorbate;Sorbate, Potassium;Sorbate, Sodium;Sorbic Acid



 Xi
Xi

