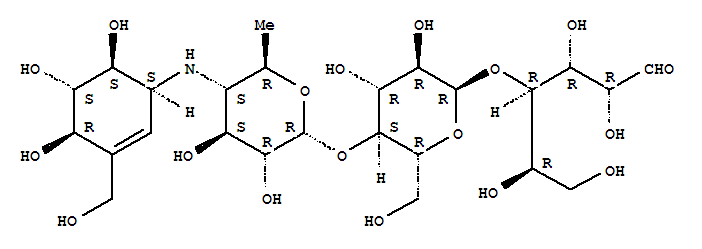
- Chemical Name:Precose
- CAS No.:56180-94-0
- Molecular Formula:C25H43NO18
- Molecular Weight:645.612
- Hs Code.:29400090
- European Community (EC) Number:260-030-7
- Nikkaji Number:J1.629.106D
- Wikipedia:Acarbose
- Wikidata:Q338005
- NCI Thesaurus Code:C983
- RXCUI:16681
- Pharos Ligand ID:2GHRKRPZGFS5
- ChEMBL ID:CHEMBL1566
- Mol file:56180-94-0.mol
Synonyms:Acarbose;Bay g 5421;Glucobay;Glucor;Glumida;Prandase;Precose