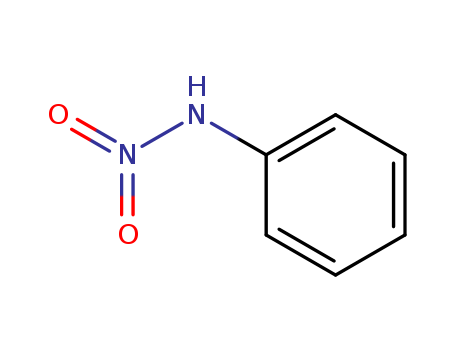
- Chemical Name:Nitroaniline
- CAS No.:645-55-6
- Molecular Formula:C6H6 N2 O2
- Molecular Weight:138.126
- Hs Code.:2928000090
- DSSTox Substance ID:DTXSID80214749
- Wikidata:Q83090646
- ChEMBL ID:CHEMBL96807
- Mol file:645-55-6.mol
Synonyms:N-Nitroaniline;NITROANILIDE;645-55-6;N-Nitroaniline [Forbidden];oxyazoxybenzene;BENZENAMINE, N-NITRO-;Nitro aniline;3-nitroaminobenzene;Aniline, N-nitro-;SCHEMBL6001;CHEMBL96807;DTXSID80214749;BDBM50473789