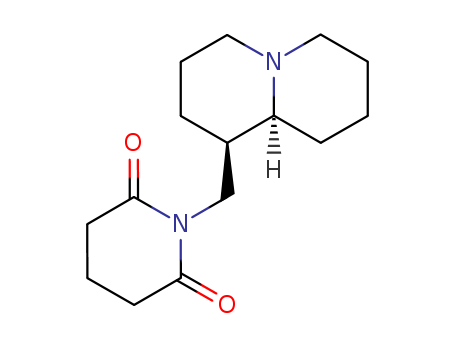
- Chemical Name:Lamprolobine
- CAS No.:18688-40-9
- Molecular Formula:C15H24N2O2
- Molecular Weight:264.368
- Hs Code.:2925190090
- DSSTox Substance ID:DTXSID00171946
- Nikkaji Number:J1.076.775J
- Wikidata:Q27107171
- Metabolomics Workbench ID:67969
- Mol file:18688-40-9.mol
Synonyms:Lamprolobine;18688-40-9;Lamprolobin;1-[[(1R,9aR)-2,3,4,6,7,8,9,9a-octahydro-1H-quinolizin-1-yl]methyl]piperidine-2,6-dione;(1R-cis)-1-((Octahydro-2H-quinolizin-1-yl)methyl)-2,6-piperidinedione;1-(((1R,9aR)-octahydro-2H-quinolizin-1-yl)methyl)piperidine-2,6-dione;2,6-Piperidinedione, 1-((octahydro-2H-quinolizin-1-yl)methyl)-, (1R-cis)-;AC1L3EBU;AC1Q6FAJ;2,6-Piperidinedione, 1-[(octahydro-2H-quinolizin-1-yl)methyl]-, (1R-cis)-;SureCN40596;SCHEMBL40596;CHEBI:6369;DTXSID00171946;AKOS040752400;Q27107171