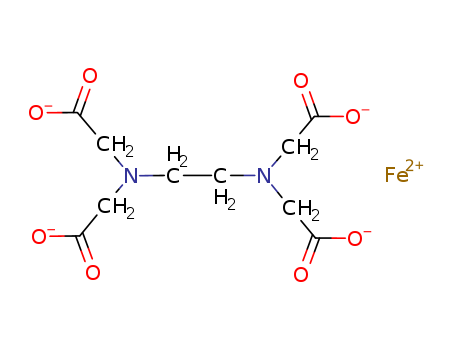
- Chemical Name:Iron(II)-edta
- CAS No.:15651-72-6
- Molecular Formula:C10H12 Fe N2 O8
- Molecular Weight:344.061
- Hs Code.:2922499990
- Wikidata:Q27113964
- Mol file:15651-72-6.mol
Synonyms:EDTA Fe(II);Fe(II)-EDTA;Fe(II)-EDTA, diammonium salt;Fe(II)-EDTA, dihydrogen;Fe(II)-EDTA, dipotassium salt;Fe(II)-EDTA, disodium salt;Fe(II)-EDTA, sodium-hydrogen salt;iron(II)-EDTA