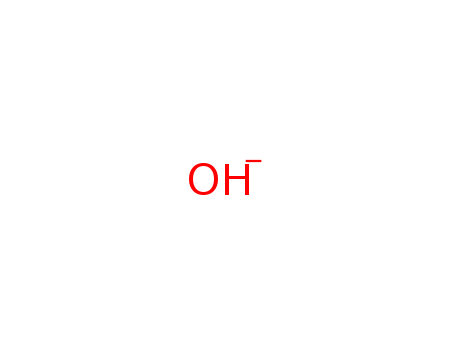
Chemical Property of Hydroxide
Edit
Chemical Property:
- Vapor Pressure:24.5mmHg at 25°C
- Boiling Point:100°Cat760mmHg
- Flash Point:°C
- PSA:23.06000
- Density:g/cm3
- LogP:-0.17680
- XLogP3:-0.6
- Hydrogen Bond Donor Count:1
- Hydrogen Bond Acceptor Count:1
- Rotatable Bond Count:0
- Exact Mass:17.002739651
- Heavy Atom Count:1
- Complexity:0
- Purity/Quality:
-
99% *data from raw suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
SDS file from LookChem
Useful:
- Canonical SMILES:[OH-]
-
Uses
Hydroxide ions are released by the resin as anions are adsorbed from the liquid phase. The effect is elimination of acidity in the liquid and conversion of the resin to a salt form. Typically, the resin is restored to the OH? form with a 4% solution of NaOH. The hydroxide form is also used in salt splitting applications. resin ? N(CH3)3OH-+Na+ + Cl- ? resin ? N(CH3)3Cl- + Na+ + OH- Salt forms of a strong base anion exchangerare used to remove other anions for which the resin has greater selectivity. 2 resin ? N(CH3)3Cl- +2Na+SO42- ? (resin ? N(CH3)3)2SO42- +2 Na+ +2Cl-