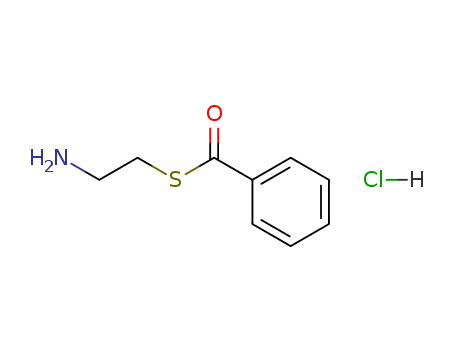
- Chemical Name:Thiobenzoic acid S-2-aminoethyl ester hydrochloride
- CAS No.:17612-90-7
- Molecular Formula:C9H12ClNOS
- Molecular Weight:217.72
- Hs Code.:2930909090
- Mol file:17612-90-7.mol
Synonyms:Thiobenzoic acid S-2-aminoethyl ester hydrochloride;2-benzoylsulfanylethylazanium;chloride;AI3-52529;S-Benzoyl-2-mercaptoethylamine hydrochloride;Benzenecarbothioic acid, S-(2-aminoethyl) ester, hydrochloride;BENZOIC ACID, THIO-, S-2-AMINOETHYL ESTER, HYDROCHLORIDE;LS-38322