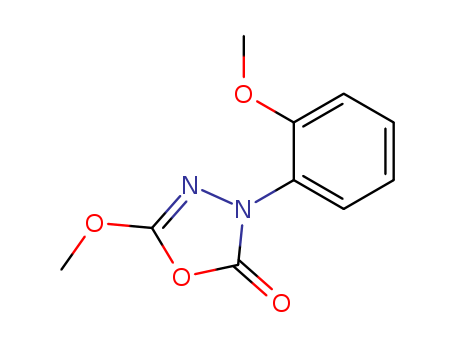
- Chemical Name:Metoxadiazone
- CAS No.:60589-06-2
- Molecular Formula:C10H10 N2 O4
- Molecular Weight:222.2
- Hs Code.:
- UNII:15Z0OMV86O
- DSSTox Substance ID:DTXSID50866815
- Nikkaji Number:J65.683F
- Wikidata:Q27155119
- Mol file:60589-06-2.mol
Synonyms:3-(2-methoxyphenyl)-5-methoxy-1,3,4-oxadiazol-2(3H)-one;MPOX