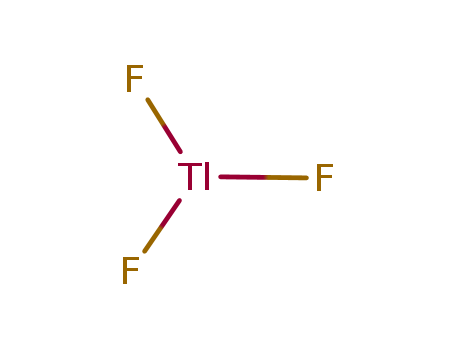
- Chemical Name:Thallic fluoride
- CAS No.:7783-57-5
- Molecular Formula:F3Tl
- Molecular Weight:261.378
- Hs Code.:
- UNII:ZJ85SF5XFJ
- Nikkaji Number:J134.298C
- Mol file:7783-57-5.mol
Synonyms:THALLIC FLUORIDE;thallium(3+) trifluoride;THALLIUM TRIFLUORIDE [MI];A839220


 T+,
T+, N,
N, T
T


