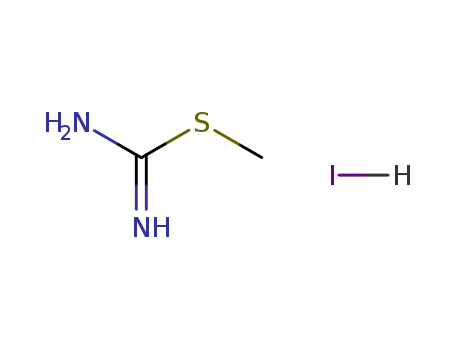
- Chemical Name:Methyl aminomethanimidothioate hydroiodide
- CAS No.:4338-95-8
- Molecular Formula:C2H6 N2 S . H I
- Molecular Weight:218.061
- Hs Code.:2930909090
- European Community (EC) Number:224-391-4
- NSC Number:142512
- Mol file:4338-95-8.mol
Synonyms:4338-95-8;Methyl aminomethanimidothioate hydroiodide;Sulfiode;methyl carbamimidothioate hydroiodide;methyl carbamimidothioate;hydroiodide;Methylthiouronium iodide;S-Methylthiuronium iodide;S-Methylthiouronium iodide;S-Methylisothiouronium iodide;2-Methyl-2-thiopseudourea hydroiodide;2-Methylisothiouronium iodide;S-Methylisothiourea hydriodide;EINECS 224-391-4;NSC 142512;C2H6N2S.HI;AI3-62929;Psuedourea, 2-methyl-2-thio-, monohydriodide;Carbamimidothioic acid, methyl ester, monohydriodide;(methylsulfanyl)methanimidamide hydroiodide;Pseudourea, 2-methyl-2-thio-, hydriodide;S-Methylisothiourea sulfate;C2-H6-N2-S;2-Methyl-isothiourea;hydriodide;2-Methyl-isothiourea HI;SCHEMBL916721;S-Methylisothiourea hydroiodide;2-Methyl-isothiourea hydriodide;S-methyl-isothiourea hydroiodide;S-Methylpseudothioharnstoffhydrojodid;MFCD00035598;NSC142512;Carbamimidothioic acid, monohydriodide;AKOS015996770;NSC-142512;AS-19809;METHYL CARBAMIMIDOTHIOATEHYDROIODIDE;S-methylisothiopseudouronium monohydroiodide;LS-126192;CS-0199730;FT-0635238;METHYLAMINOMETHANIMIDOTHIOATEHYDROIODIDE;EN300-412490;A826261;J-522587;methyl aminomethanimidothioate hydroiodide, AldrichCPR;Carbamimidothioic acid, methyl ester, monohydriodide (9CI);2-methylisothiourea hydroiodide