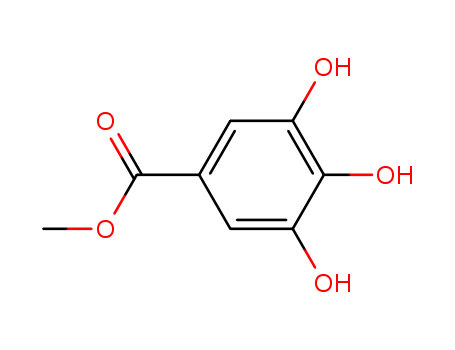
- Chemical Name:Methyl gallate
- CAS No.:99-24-1
- Molecular Formula:C8H8O5
- Molecular Weight:184.149
- Hs Code.:2918.29
- European Community (EC) Number:202-741-7
- NSC Number:363001
- UNII:623D3XG80C
- DSSTox Substance ID:DTXSID3059189
- Nikkaji Number:J4.967K
- Wikipedia:Methyl_gallate
- Wikidata:Q15425833
- Metabolomics Workbench ID:143431
- ChEMBL ID:CHEMBL65675
- Mol file:99-24-1.mol
Synonyms:gallic acid, methyl ester;methyl 3,4,5-trihydroxybenzoate;methyl gallate


 Xn,
Xn, Xi
Xi


