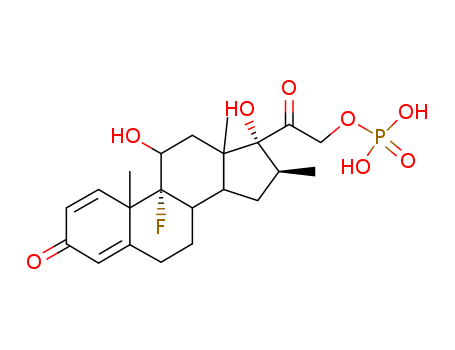
- Chemical Name:Betamethasone phosphate
- CAS No.:360-63-4
- Molecular Formula:C22H30FO8P
- Molecular Weight:472.448
- Hs Code.:
- European Community (EC) Number:206-636-7
- UNII:YJO1F9W10R
- DSSTox Substance ID:DTXSID2047224
- Nikkaji Number:J193.862B
- Wikipedia:Betamethasone_phosphate
- Wikidata:Q27137041
- NCI Thesaurus Code:C77406
- Pharos Ligand ID:UWRLU1M46J7K
- Metabolomics Workbench ID:65116
- ChEMBL ID:CHEMBL1201207
- Mol file:360-63-4.mol
Synonyms:Bentelan;betamethason sodium phosphate;betamethasone 21-phosphate;betamethasone disodium phosphate;betamethasone disodium phosphate, (11beta)-isomer;betamethasone phosphate;betamethasone sodium phosphate;betamethasone sodium phosphate, (11beta,16beta)-isomer;Betnesol;Celestone phosphate;Rinderone