- Chemical Name:Zincchloride
- CAS No.:7646-85-7
- Deprecated CAS:53917-99-0
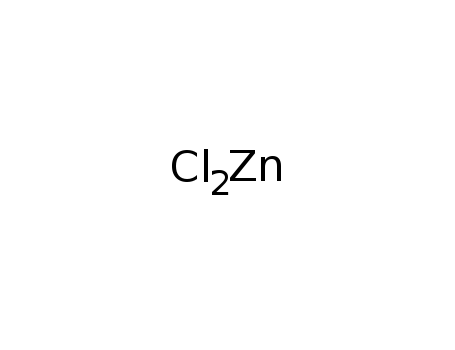
- Molecular Formula:ZnCl2
- Molecular Weight:136.296
- Hs Code.:2827.36 Oral rat LD50: 350 mg/kg
- UNII:86Q357L16B
- DSSTox Substance ID:DTXSID2035013
- Nikkaji Number:J3.744C
- Pharos Ligand ID:VRNNW5CV2L8L
- ChEMBL ID:CHEMBL1200679
- Mol file:7646-85-7.mol
Synonyms:zincchloride;zinc;dichloride;Zincum muriaticum;CHEBI:49976;DTXSID2035013;zinc-chloride;zink chloride;Galvanizers Flux;zinc(II)chloride;zinc(II)-chloride;zinc (II) chloride;FLUX LOTOP;D05ELV;Epitope ID:156810;ZINC CHLORIDE [MI];ZINC CHLORIDE [JAN];ZINC CHLORIDE [HSDB];ZINC CHLORIDE [INCI];ZINC CHLORIDE [WHO-DD];CHEMBL1200679;ZINCUM MURIATICUM [HPUS];JIAARYAFYJHUJI-UHFFFAOYSA-L;ZINC CHLORIDE [ORANGE BOOK];Tox21_301492;Zinc Chloride, ACS grade -10 mesh;AKOS024438088;DB14533;NCGC00255612-01;CAS-7646-85-7;Z0011;Z0014;Z0019;Z0020;Z0038;Zinc chloride, trace metals grade, 99.99%;Zinc chloride, anhydrous free-flowing, reagent grade;Zinc Chloride (ca. 6.5% in Ethyl Ether, ca. 0.4mol/L);Zinc chloride, ultra dry, -10 mesh beads, ampoule, 99.99% trace metals grade


 Xn,
Xn, C,
C, N
N
 C:Corrosive;
C:Corrosive;

