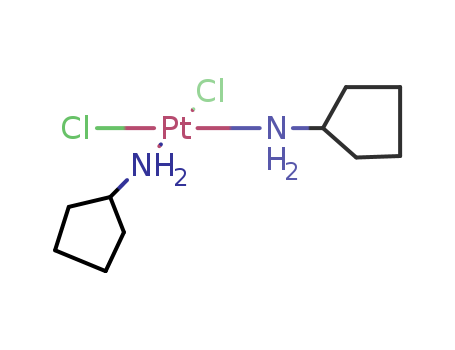
- Chemical Name:Dichlorobis(cyclopentylamine)platinum
- CAS No.:38780-36-8
- Molecular Formula:C10H22Cl2N2Pt
- Molecular Weight:436.284
- Hs Code.:
- European Community (EC) Number:254-125-2
- UNII:X4G53V7WUH
- DSSTox Substance ID:DTXSID00972273
- Mol file:38780-36-8.mol
Synonyms:cis-dichlorobis(cyclopentylamine)platinum;dichlorobis(cyclopentylamine)platinum;dichlorobis(cyclopentylamine)platinum, (SP-4-1)-isomer;dichlorobis(cyclopentylamine)platinum, 195Pt-labeled, (SP-4-2)-isomer;NSC 170898