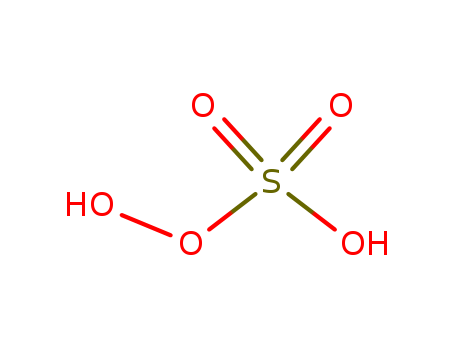
Chemical Property of peroxomonosulphuric acid
Edit
Chemical Property:
- Melting Point:45, decomposing [HAW93]
- PKA:pK2 of Caros acid 9.4 ± 0.1(at 25℃)
- PSA:79.44000
- Density:2.239g/cm3
- LogP:0.13700
- XLogP3:-1.3
- Hydrogen Bond Donor Count:2
- Hydrogen Bond Acceptor Count:5
- Rotatable Bond Count:1
- Exact Mass:113.96229433
- Heavy Atom Count:6
- Complexity:100
- Purity/Quality:
-
98%Min *data from raw suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
SDS file from LookChem
Useful:
- Canonical SMILES:OOS(=O)(=O)O
-
Physical properties
White crystalline solid; unstable, decomposes at 45°C; commercial product is a syrupy liquid containing equal parts of Caro’s acid and sulfuric acid; stored at dry ice temperature; very soluble in water.
-
Uses
peroxomonosulphuric acid (Caro's acid) is used in the preparation of dyes and bleaching agents. It also is used as a strong oxidizing reagent to convert ketones to lactones, to convert olefins to glycols and esters, and to analyse pyridine, aniline and many alkaloids. In preparation of dyes; oxidation of olefins to a-glycols; oxidation of ketones to lactones or esters; treating woolens to prevent felting and shrinking; in bleaching compositions. Peroxymonosulfuric acid is used as an oxidizingagent to make glycols, lactones, and esters; formaking dyes; and in bleaching composition.