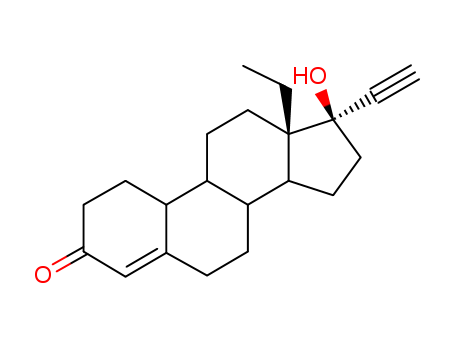
- Chemical Name:(+)-Norgestrel
- CAS No.:797-64-8
- Deprecated CAS:76497-12-6,150200-16-1,150200-16-1
- Molecular Formula:C21H28O2
- Molecular Weight:312.452
- Hs Code.:
- European Community (EC) Number:686-277-4
- UNII:Z2QG2679YT
- DSSTox Substance ID:DTXSID90892173
- Nikkaji Number:J76.208C
- Wikidata:Q27122247
- Metabolomics Workbench ID:57563
- Mol file:797-64-8.mol
Synonyms:dextronorgestrel;dextronorgestrel, (8alpha,17alpha)-(+-)-isomer