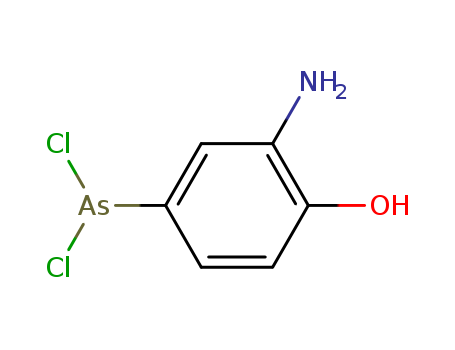
- Chemical Name:Dichlorophenarsine
- CAS No.:455-83-4
- Molecular Formula:C6H6AsCl2NO
- Molecular Weight:253.947
- Hs Code.:2931900090
- UNII:3WD5400T9N
- DSSTox Substance ID:DTXSID10871748
- Nikkaji Number:J5.757F
- Wikidata:Q27258133
- NCI Thesaurus Code:C75247
- Mol file:455-83-4.mol
Synonyms:Dichlorophenarsine;455-83-4;dichloromapharside;3WD5400T9N;Diclorofenarsina;2-amino-4-dichloroarsanylphenol;Dichlorphenarsinum;Dichlorophenarsinum;UNII-3WD5400T9N;Dichlorophenarsine [INN:BAN];Diclorofenarsina [INN-Spanish];SCHEMBL1230304;DICHLOROPHENARSINE [INN];Dichlorophenarsinum [INN-Latin];DTXSID10871748;CHEBI:135036;(3-Amino-4-hydroxyphenyl)arsonous dichloride;Arsonous dichloride, (3-amino-4-hydroxyphenyl)-;Q27258133