- Chemical Name:Pyrvinium
- CAS No.:7187-62-4
- Molecular Formula:C26H28N3
- Molecular Weight:382.528
- Hs Code.:
- UNII:6B9991FLU3
- ChEMBL ID:CHEMBL1201303
- DSSTox Substance ID:DTXSID2043795
- Metabolomics Workbench ID:70135
- NCI Thesaurus Code:C77330
- Pharos Ligand ID:MLS4NVXQURYK
- Wikidata:Q264039
- Wikipedia:Pyrvinium
- Mol file:7187-62-4.mol
Synonyms:Molevac;Pamoxan;Povanyl;Pyrcon;pyrvinium;pyrvinium iodide;pyrvinium monohydroxide;pyrvinium pamoate;pyrvinium pamoate (2:1);Vankin;Vanquin

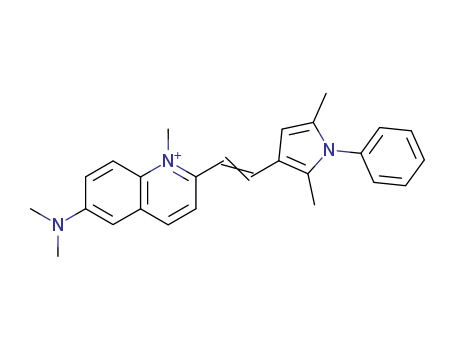
![N,N-dimethyl-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-6-quinolinamine](/Databaselist/images/loading.webp)


