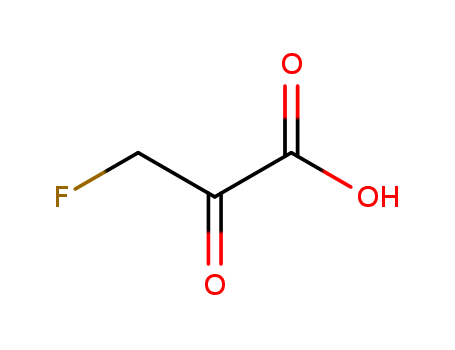
- Chemical Name:3-Fluoro-2-oxopropanoic acid
- CAS No.:433-48-7
- Molecular Formula:C3H3 F O3
- Molecular Weight:106.053
- Hs Code.:2918300090
- NSC Number:21734
- UNII:040OM3QF5R
- DSSTox Substance ID:DTXSID30962985
- Nikkaji Number:J49.680D
- Wikidata:Q27124338
- Metabolomics Workbench ID:58882
- ChEMBL ID:CHEMBL1162543
- Mol file:433-48-7.mol
Synonyms:3-fluoropyruvate;3-fluoropyruvate, sodium salt