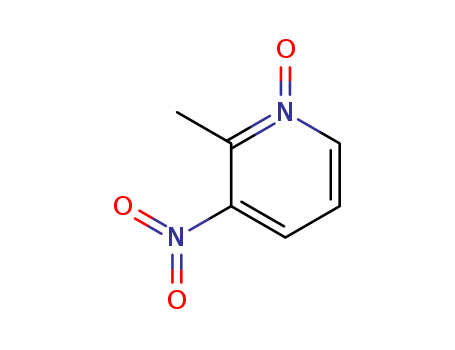
- Chemical Name:2-Methyl-3-nitropyridine N-oxide
- CAS No.:5236-76-0
- Molecular Formula:C6H6 N2 O3
- Molecular Weight:154.125
- Hs Code.:2933399090
- Mol file:5236-76-0.mol
Synonyms:2-Picoline,3-nitro-, 1-oxide (7CI,8CI); 2-Methyl-3-nitropyridine 1-oxide;2-Methyl-3-nitropyridine N-oxide